pH Levels Matter!
pH levels are crucial when using hypochlorous acid (HOCl) because they directly affect the stability and efficacy of the acid as a disinfectant and sanitizer. Here are the key reasons why pH levels matter:
- Stability of HOCl:
- Hypochlorous acid is most stable and effective in a slightly acidic to neutral pH range (around pH 5-7.5). Outside this range, HOCl can dissociate into less effective forms.
- At low pH levels (below 5), HOCl can convert into chlorine gas, which is less effective and can be hazardous.
- At high pH levels (above 7.5), HOCl dissociates into hypochlorite ions (OCl⁻), which are significantly less effective as a disinfectant.
- Disinfectant Efficacy:
- The antimicrobial activity of hypochlorous acid is highest at pH levels where HOCl predominates. In the optimal pH range, HOCl can effectively penetrate microbial cell walls and disrupt their functions, leading to the death of bacteria, viruses, and other pathogens.
- As the pH increases and HOCl converts to OCl⁻, the disinfectant power decreases. Hypochlorite ions are less effective in killing microbes.
- Safety:
- Maintaining the appropriate pH ensures the safe use of hypochlorous acid solutions. At very low pH levels, the production of chlorine gas can pose inhalation risks.
- At higher pH levels, although less effective, the solution is less likely to produce harmful byproducts.
- Environmental Impact:
- Using HOCl within the appropriate pH range minimizes the formation of harmful byproducts like chloramines and trihalomethanes, which can occur at certain pH levels and are environmentally detrimental.

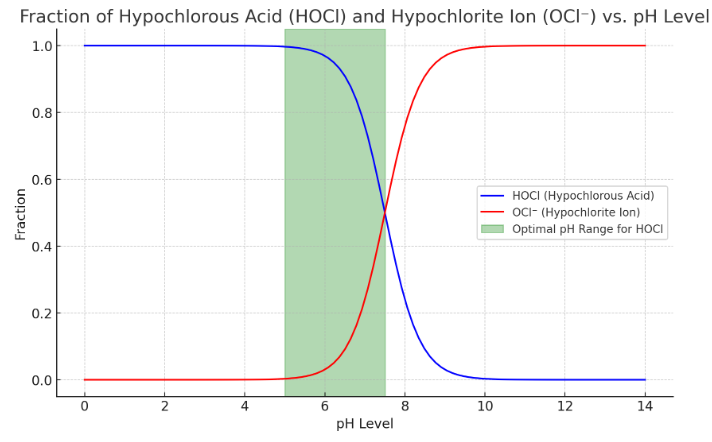
Here's a graph illustrating the fractions of hypochlorous acid (HOCl) and hypochlorite ion (OCl⁻) as a function of pH levels. The blue curve represents the fraction of HOCl, while the red curve represents the fraction of OCl⁻.
The green shaded area highlights the optimal pH range (5 to 7.5) for HOCl, where it is most stable and effective as a disinfectant. As the pH increases beyond this range, HOCl dissociates into OCl⁻, which is less effective as a disinfectant. Conversely, at very low pH levels, there is a risk of chlorine gas formation.
Our disinfectant technology products are tested on a regular basis using chlorine test strips to adjust to the desired chlorine level for sanitizing, deodorizing and cleaning applications.
In summary, controlling the pH levels when using hypochlorous acid is essential for ensuring its effectiveness as a disinfectant, maintaining safety, and minimizing environmental impact.
